TB Testing Kit: Now, TB testing will be done at a lower cost than indigenous kit; ICMR has approved it.
- bySherya
- 06 Oct, 2025

TB Symptoms: TB is an infectious disease that primarily affects the lungs. Early and accurate diagnosis is crucial so that patient treatment can begin in a timely manner.
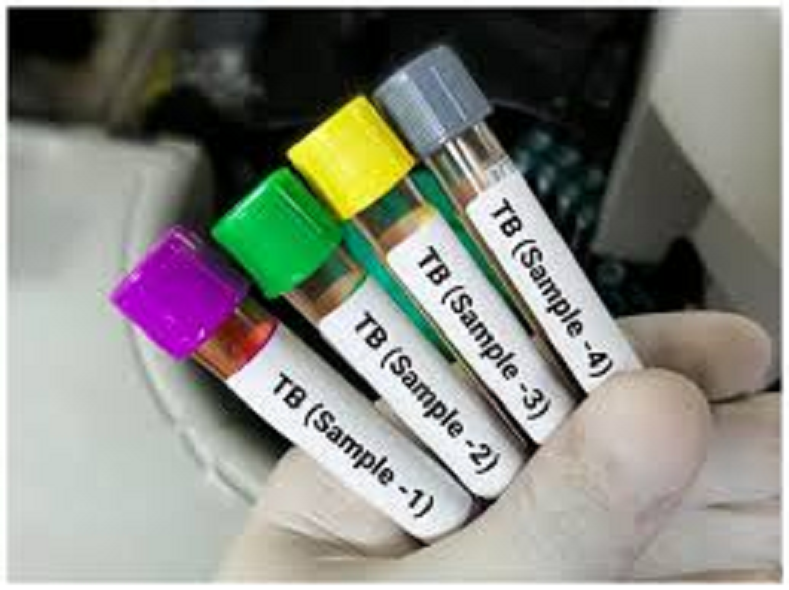
An important step has been taken to make TB (tuberculosis) testing easier, faster, and cheaper in the country. In fact, the Indian Council of Medical Research (ICMR) has approved the indigenous "Quantiplus MTB Fast Detection Kit," developed by Telangana-based company Huwel Lifesciences. The kit's unique feature is that it can test 96 samples simultaneously in a very short time. This kit can also reduce testing costs by approximately 20%. This kit is the first open-system RT-PCR test for detecting pulmonary TB, which will help improve TB testing in the country.
This kit will revolutionize TB testing.
TB is an infectious disease that primarily affects the lungs. Early and accurate diagnosis is crucial to ensure timely treatment and prevent the spread of the disease. If a TB patient fails to seek timely treatment, they can pose a threat to those around them. Therefore, it is crucial to make TB testing easy and affordable.
How special is the new kit?
The key advantage of this new kit from Huwel Lifesciences is that it can be used with any standard PCR machine. It doesn't require any special or expensive platform. Laboratories across the country will be able to use this kit to rapidly test for TB. This kit can be easily installed in government hospitals and testing centers, significantly increasing the speed of TB testing. It will also save the cost of expensive equipment.
What will be the benefit of the new kit?
Dr. Nivedita Gupta, head of the ICMR's Communicable Diseases Division, explained that this new kit has been developed by upgrading existing devices such as TrueNat and PathoDetect. This allows for the ease of performing nucleic acid amplification tests (NAAT) on a small scale. This means that even small testing centers can now accurately diagnose TB. This kit can detect not only common TB but also drug-resistant TB (TB that does not respond to drugs).
A tongue swab will also be used for testing.
ICMR has approved another new technology from Huwel Lifesciences, called the UniAmp MTB Nucleic Acid Test Card. This kit can test for TB using tongue swabs (saliva) instead of saliva. This is especially beneficial for children and the elderly, as collecting saliva samples is often difficult. Traditional TB testing requires sputum samples to undergo complex procedures, but this new kit will simplify and speed up the testing process.